Spotlight 🔍 Ocumetics Technology
Investors Eye Ocumetics’s Clear Line
of Sight to a Multi-Billion-Dollar Market
- Ocumetics is delivering clinical proof in first-in-human surgeries, showing functional vision without glasses and validating a breakthrough technology in a multi-billion-dollar medical market.
- Clinical outcomes are showing the Ocumetics Accommodating Lens can deliver the natural focusing ability strengthening confidence in commercial uptake.
- The company is moving into a pivotal commercialization phase with FDA trials planned for 2027 and early revenue expected soon after, as valuation continues to rise with each milestone achieved.
“Almost everyone develops cataracts if they live long enough or they become a caregiver of a cataract patient … you do not get a pass on that … So when we talk about this opportunity, we are talking about a guaranteed, recurring market that is already more than $4.5 billion globally, and growing every year as populations age.”
— Dean Burns, President and CEO of Ocumetics Technology
It starts quietly. A longer arm to read the menu. Your phone display looks smaller each year. You squint at a label and wonder when things changed. For many, these are the early signs of aging we’d rather ignore. But eventually, for every person who lives long enough, vision loss from cataracts will make the world fade.
Every year, more than 30 million people around the world undergo cataract surgery. It is one of the most common medical procedures on the planet. Yet even after this highly advanced surgery, most patients still need reading glasses or bifocals for everyday life.
That is the starting point for Ocumetics Technology Corp. (TSXV: OTC), a Canadian medical-technology company that believes cataract surgery should not only restore clarity but also restore how vision is supposed to work.
“The company spent the lion’s share of its early years doing research on the anatomy of the eye and translated that into how to build an intraocular lens that gives a patient the ability to see distance, intermediate and near. We call that accommodating,” said Dean Burns, President and CEO of Ocumetics.
The result is the Ocumetics Accommodating Lens, a tiny, engineered implant placed during a routine 15-to-20-minute cataract procedure. Instead of leaving patients dependent on glasses, the lens is meant to restore natural, hands-free sight.
Burns puts it bluntly: “Once this technology is proven you will not need glasses anymore.”
And the goal is not limited to perfect vision in perfect settings. It is day-to-day sight. Reading a phone. Driving at night. Looking someone in the eye without adjusting your eyewear.
And proving that performance in real patients is now well underway.
Ocumetics is currently advancing its first-in-human clinical study in Mexico City, where surgeons are implanting the lens in cataract patients under rigorous monitoring. Burns says the location was strategic. The high population density ensures fast enrolment, helping generate meaningful data in a fraction of the time typical North American trials require.
“So far the patients are doing extremely well,” said Burns. “With devices, you know right away whether it works or not. It either fixes the problem or it makes it worse.”
For Ocumetics, the early signs are exactly what the company hoped to see and what investors have been waiting for.
The company recently reported its first batch of post-operative results, confirming that the lens is performing safely and helping patients achieve functional vision without glasses. It’s a critical milestone that shifts the company firmly into its commercialization runway, turning years of research into visible, real-world benefit.
That progress is happening in a market where demand is not theoretical, seasonal, or optional. It is built into human biology.

Every cataract surgery requires a replacement lens. And with more than 30 million procedures performed globally each year, that need only increases as populations age. Yet even in well-resourced health-care systems, the outcome still leaves most patients dependent on glasses for daily activities.
Traditional lenses currently in use restore essential clarity, but rarely restore the full focusing function we enjoyed before our eyes aged. They give back vision, but not freedom.
The Ocumetics Accommodating Lens is designed to behave like the natural lens of a younger eye, shifting seamlessly through near, intermediate and far vision. If the company continues to deliver the clinical performance seen in its early human data, patients may not just see clearly, they may see normally again, without glasses.
A Top Pick in Muskoka
TSXV: OTC
Market Cap
Price¹
Picked²
- As of market open on October 31, 2025.
- As of market open on Monday, September 29 after being selected as a Top Pick at the CEM Muskoka Capital Event.
In this Q&A, Dean Burns, President and CEO of Ocumetics Technology Corp, outlines the company’s path to commercialization following its Top Pick recognition at the CEM Muskoka Capital Event 2025.
What are the Mexico City clinical trials showing so far?
“The reason we went to Mexico City is because the population density is so high that recruiting is not an issue. Early outcomes are validating what the engineering promised … patients are recovering well and showing functional vision without glasses. Underpinning our clinical confidence is a major performance breakthrough demonstrated before the lens ever entered the eye. In bench testing, the lens’s accommodative response, which is its ability to shift focus from near to far, hit up to +14 diopters, far beyond the +2.5 to +4 diopters a young human eye typically needs for everyday tasks like reading and driving. Our design has overshot expectations.”
What is Ocumetics Total Addressable Market?
“When you drill down into the lens segment, it is a $4.5-billion market, which is growing as the population ages. Whether you invest in the company or not, everyone will either be a patient or a caregiver of a patient who has a cataract. That makes the demand guaranteed, not optional. We take out a cataract and replace it with a prosthetic artificial lens, giving you the same vision that you have when you are about 30 years old. This positions Ocumetics squarely in the premium cataract lens category, where patients and surgeons actively choose the best visual outcomes. My estimate is we will take over about 60% of that market.”
Why invest in Ocumetics now?
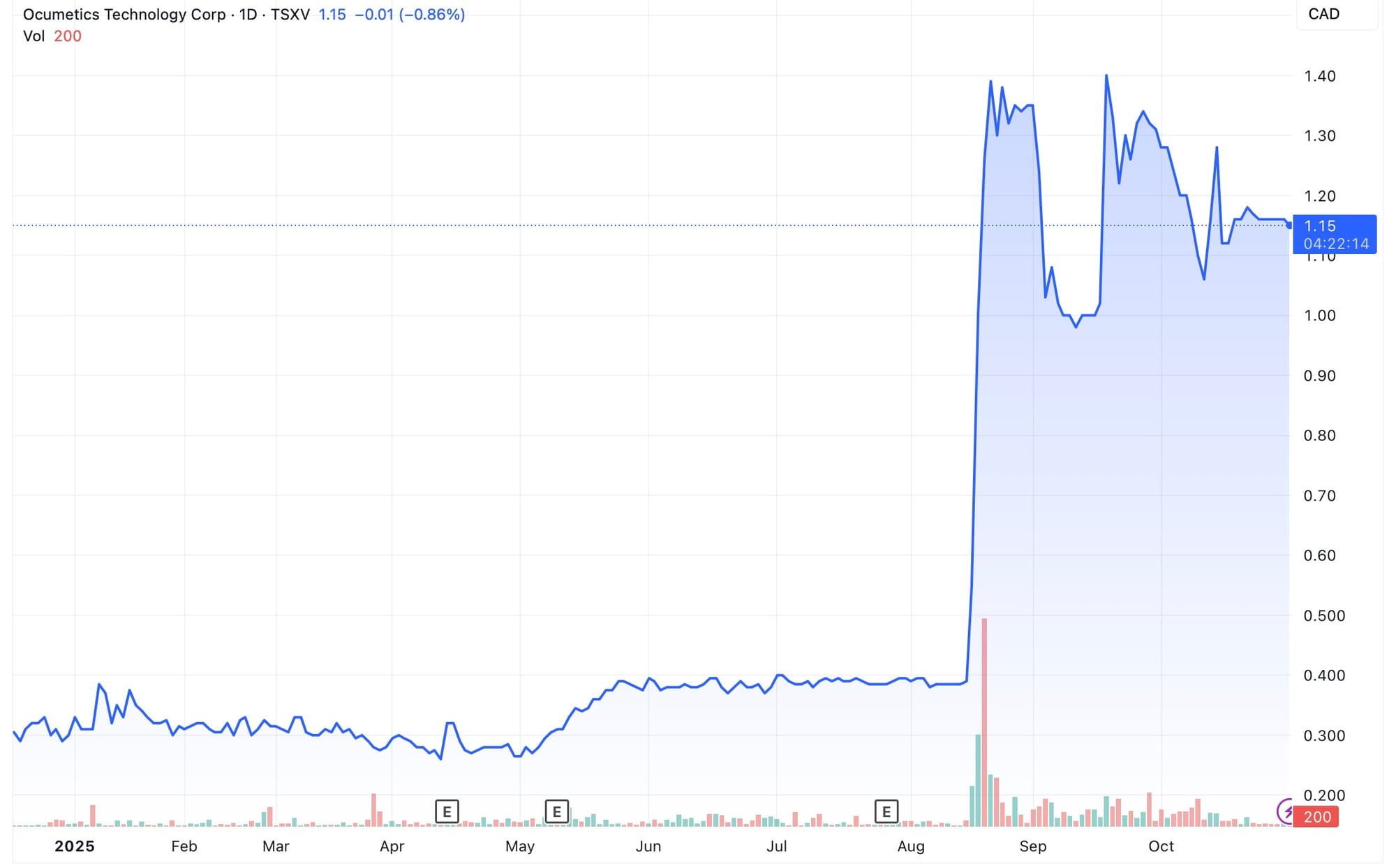
“Once we announced we were first in human trials, our stock went from 45 cents all the way up to two dollars. Once we get to the pivotal trial, the value of the company goes up significantly. The next 18 months are about expanding the first-in-human dataset, adding more surgeries, and reporting ongoing post-operative results that continue to demonstrate both safety and functional performance in real patients. By the end of 2026, the company expects to complete this clinical phase and move straight into regulatory preparation, setting the stage for its U.S. path. 2027 is a big year for us. That is when the company anticipates receiving approval to begin its pivotal FDA trial, a major step toward full market access in the United States. As that trial launches, Ocumetics also expects to secure its first commercial approvals outside the U.S., allowing early revenue generation to begin in initial launch markets.”
Our View
- Clinical Proof that Reduces Risk
Early human data from Mexico City confirms what years of engineering predicted: patients are recovering well and achieving functional vision without glasses. With medical devices, results are immediate, which means Ocumetics is already demonstrating success in a real clinical setting, which is a major de-risking event for investors. - Guaranteed, Expanding Market with Superior Solution
More than 30 million cataract surgeries occur every year and everyone requires a lens, demand that grows as the world ages. Conventional options restore clarity but not natural focusing ability, leaving people dependent on glasses. Ocumetics is positioned to lead the premium lens market by restoring true accommodation. - Commercialization Runway with Visible Value Catalysts
Shares already jumped from 45 cents to two dollars after first-in-human results, showing how closely valuation tracks execution. With a pivotal FDA trial planned in 2027 and early revenue expected outside the U.S. soon after, the company is entering a cycle of milestone-driven upside as it advances toward global market access.
Success in first in human trials often draws investments from world leading technology companies. CEO Dean Burns has recently met with four of the top seven eye care technology companies in the world, on their invitation.
Weekly Insight
Each week, CEM Partner and Portfolio Manager Ryan Iverson spotlights the ideas and companies sparking investor interest from emerging growth stories to the Top Picks featured across CEM’s Capital Events. This series brings real insights from the innovators shaping tomorrow’s markets and reveals where investors are finding the next breakout opportunities.

2026 CEM Event Schedule
Register today to see if you qualify to attend!
AlphaNorth Capital Event (Bahamas): January 16-18, 2026
Whistler Capital Event: February 6-8, 2026
Scottsdale Capital Event: April 10-12, 2026
Bermuda Capital Event: June 12-14, 2026
TSX Venture Growth Capital Event (Okanagan): July 17-19, 2026
Muskoka Capital Event: September 25-27, 2026
Stay informed. Stay ahead. Stay with the Investor Breakout Exchange:
X | Facebook | LinkedIn | Instagram | YouTube | TikTok
Warm Regards and Happy Investing,
Fabian Dawson
